Abstract
Introduction: As there is limited evidence to guide management of patients with steroid-refractory graft-versus-host disease (SR-GVHD) there is a broad variability of clinical practices. To document the current practice and assess the impact of emerging new drugs in SR-GVHD, Transplant Complications Working Party (TCWP) of the EBMT performed a survey among EBMT centers that covered specific treatment decisions on first- and second-line treatment of acute and chronic GVHD (aGVHD and cGVHD).
Methods: The survey was conducted from December 2020 to March 2021 among EBMT centers. A questionnaire was developed by the study authors and used for data collection. It consisted of 40 questions focused on general approach in the first-line treatment, preferred second-line treatment in SR-GVHD and ancillary care in aGVHD and cGVHD.
Results: 145 centers from 33 countries agreed to participate and responded to the questionnaire.
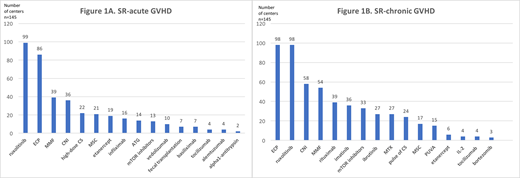
First-line treatment of aGVHD was reported as rather homogenous; with most centers (68%) starting with lower doses of corticosteroids (CS) (<2mg/kg) in lower grade aGVHD (grade 2a) and most centers (88%) starting with higher doses (2mg/kg) in aGVHD grades >2b. On the other hand, the evaluation of response to CS was more heterogeneous: at 3 days in 33%, at 5 days in 30%, at 7 days in 15% of centers and depending on severity of aGVHD in most other centers. In the presence of SR-aGVHD, 50% of centers consider inclusion of patients in clinical trials. Although as much as 85% of centers reported to have a standard operating procedure (SOP) for SR-aGVHD management, only 45% (n=66) have an established one or 2-agent second-line treatment; most frequently ruxolitinib (n=46) and/or extracorporeal photopheresis (ECP) (n=29). All other centers reported a very heterogeneous practice and listed multiple agents (range, 3-10) as second-line treatment options. In total, the most used agents for SR-aGVHD as shown in Figure 1A are ruxolitinib in 68%, ECP in 59%, mycophenolate-mofetil (MMF) in 27%, calcineurin inhibitors (CNI) in 25%, high-dose CS (>2mg/kg) in 15%, mesenchymal stem cells (MSC) in 14%, etanercept in 13%, infliximab in 11% and anti-thymocyte globulin (ATG) in 10% of centers.
Clinical practice in first-line treatment of cGVHD again appeared relatively homogeneous; 58% of centers reported to treat mild forms with topical treatment only, unless affected organs could not be reached topically. In moderate/severe cGVHD, the majority (71%) of centers start with 0.5-1mg/kg of CS, in 45% with addition of CNI, while others use higher doses of CS +/- other agents. The evaluation of response was done before 4 weeks in 41% of centers, between 5-8 weeks in 41%, while a minority performed later response assessment or based the latter on organ affection/severity. In case of SR-cGVHD, 56% of centers would consider the inclusion in clinical trials, while only 65% have a SOP on management of these patients. One third of centers (35%) has an established multidisciplinary cGVHD team. Practices for SR-cGVHD again varied significantly, with most centers reporting on the use of more than 2 agents (range, 3-13) as second-line and with most applied agents as depicted in Figure 1B; ruxolitinib and ECP in 68% of centers, CNI in 40%, MMF in 37%, rituximab in 27%, imatinib in 25%, mTOR inhibitors in 23%, ibrutinib and methotrexate (MTX) in 19%, pulse of CS in 17%, MSC in 12% and PUVA therapy in 10% of centers.
Conclusions:In summary, this survey revealed a rather homogenous first-line management of aGVHD and cGVHD based on steroids in the majority of centers. However, when first-line fails, the definition of SR-GVHD remains highly variable and SR-GVHD is still treated with a seemingly "trial and error" approach as demonstrated by significant variability of clinical practices among EBMT centers for second-line treatment. However, in line with recently published prospective trials, ruxolitinib comes forth as one of the most used therapeutic modalities in both SR-aGVHD and cGVHD, together with already widely administered ECP. On the contrary, ibrutinib has not emerged as standard of care in this setting. Future efforts should be invested in finding a standardized approach in SR-GVHD by directly comparing most applied second-line agents in prospective trials as well as evidence-based personalized treatment approaches.
Peric: Therakos, Servier, MSD, Astellas, Novartis, Abbvie, Pfizer: Honoraria. Schoemans: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: personal fees , Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants and personal fees; Gilead: Other: travel grants; CIBMTR: Consultancy, Other: travel grants; Janssen: Membership on an entity's Board of Directors or advisory committees; BHS: Membership on an entity's Board of Directors or advisory committees, Other: travel grants and personal fees , Research Funding; Jazz Pharmaceuticals: Other: personal fees; Takeda: Other: personal fees. Koenecke: Novartis: Consultancy; Janssen: Consultancy; BMS/Celgene: Consultancy; Kite/Gilead: Consultancy; EUSA Pharm: Consultancy. Basak: Saventic Health: Current holder of individual stocks in a privately-held company. Greinix: Celgene: Consultancy; Novartis: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; Therakos: Consultancy. Penack: Omeros: Consultancy; Shionogi: Consultancy; Priothera: Consultancy; Incyte: Research Funding; Takeda: Research Funding; Therakos: Honoraria; Pfizer: Honoraria; Neovii: Honoraria; Novartis: Honoraria; MSD: Honoraria; Jazz: Honoraria; Gilead: Honoraria; Astellas: Honoraria.